Researchers at the University of Cologne and the Centre for Genomic Regulation (CRG) in Barcelona have discovered that cancer grows uniformly throughout its mass, rather than at the outer edges. The work, published today in the journal eLIFE under the title ‘High-density sampling reveals volume growth in human tumours’, questions decades-old assumptions about how the disease grows and spreads.
“We challenge the idea that a tumour is a ‘two-speed’ entity with rapidly-dividing cells on the surface and slower activity in the core. Instead, we show they are uniformly growing masses, where every region is equally active and has the potential to harbour aggressive mutations,” said Dr. Donate Weghorn, co-corresponding author of the study and researcher at the Centre for Genomic Regulation in Barcelona.
“Our findings have implications for tumour evolution. The constant churn of cells dying and being replaced by new ones throughout the tumour volume gives cancer many opportunities for evolutionary innovations, such as escaping from immune surveillance,” explained Professor Dr. Johannes Berg, co-corresponding author of the study and researcher at the University of Cologne.
For the last fifty years, researchers have hypothesized that tumours grow faster at their outer edges. Cancer cells on the surface are thought to have natural advantages compared to cells deep within. For example, peripheral cells have better access to nutrients and oxygen from surrounding healthy tissues. They can also get rid of their waste more easily.
As a tumour grows, its centre gets further and further away from the blood vessels in the area where it is growing. The cells in a tumour’s core get less and less oxygen and nutrients. The cells are also under more mechanical pressure, with compression limiting their ability to divide.
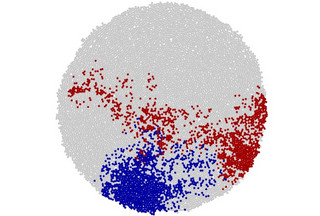
Despite this theory, the study found tumours grow throughout their mass. The researchers made the discovery thanks to spatial genomics, a technique used to study the genetic information of cells in their exact locations within a tissue. They obtained data from previous studies where hundreds of small samples were taken from different parts of liver tumours, both in two- and three-dimensional space. This provided a detailed map of the mutations throughout the tumour.
They looked at the mutations in each of the samples and developed a method to measure the direction and spread of these mutations, allowing the researchers to calculate the angles between the positions of parent cells and their mutated offspring. In the surface growth model, these angles would point outwards. Instead, the researchers found the angles were spread evenly in all directions, showing uniform growth throughout the tumour.
The study also looked at how mutations were spread within the tumour. If cancer cells grew mostly at the edges, mutations would be more clustered. They found that mutations were spread out, suggesting that cells were dividing all over the tumour.
To validate their findings further, the researchers used computer simulations to create different virtual tumours, some with surface growth and others with volume growth. The researchers compared the patterns of mutations from the simulations to the patterns found in the real tumour data. They found that mutation patterns in the real tumours matched the patterns from the volume growth simulations but not the surface growth simulations.
One of the limitations of the study is that it focused on liver cancer, so the findings might not apply universally to all types of cancer. Another limitation is that the study mainly provides insights into the early stages of tumour growth, which might not fully capture the behaviour of larger or metastatic types of cancer.
“The emergence of mutants that confer resistance to therapy are an important aspect of clinical relevance. Our work focuses on early-stage tumour growth, but expanding the research to late-arising mutations can tell us more about those mutations and why they ultimately foil many therapeutic approaches,” concluded Dr. Berg.
Media Contact:
Professor Dr Johannes Berg
Institute for Biological Physics of the University of Cologne
+49 221 470 3286
bergj@uni-koeln.de
Dr Donate Weghorn
Group leader at the Centre for Genomic Regulation, Barcelona, Spain
Press and Communications Team:
Mathias Martin
+49 221 470 1705
Publication: